Phesi and Krystelis collaborate to launch new Diversity, Equity and Inclusion Data Service for clinical development
September 7, 2023 – Clinical Trials – DEI, Krystelis, Phesi
New Diversity, Equity, and Inclusion (DEI) Data Service will harness data from 70 million patients to enable sponsors to create more diverse trials, reduce protocol amendments and meet new FDA regulations
6 September 2023 — Boston, US — Phesi, a global provider of patient-centric data software and services, today announces its partnership with Krystelis, a world leading provider of medical writing and clinical trial transparency services, to launch its new Diversity, Equity and Inclusion (DEI) Data Service. The new service harnesses data from Phesi Trial Accelerator and Krystelis’ writing services to empower sponsors to improve diversity from the trial planning stage onwards. Enabling sponsors to minimise costly protocol amendments (at an average cost of $500,000/amendment) and to meet new global DEI rules from regulators including the FDA – ensuring any therapeutic is efficacious for all intended patient populations.
Phesi’s Trial Accelerator is built on a wealth of data from 90,000 dynamically updated sources, including more than 4,000 disease indications and 70.8 million patients, with detailed data about the race and/or ethnicity of 25.9 million patients. Krystelis provides powerful medical writing capabilities that can deliver diversity-led Protocol Writing as well as Integrated Development Plans and DEI Plans. A 2022 Phesi data analysis discovered 42% of US cancer trial cohorts do not include African American patients, and almost half – 48% – have no Hispanic American patients. In a further 2023 analysis on a subset of data from 22.4 million patients, Phesi discovered only 10.2 million female patients, meaning 45% of data were collected from women.
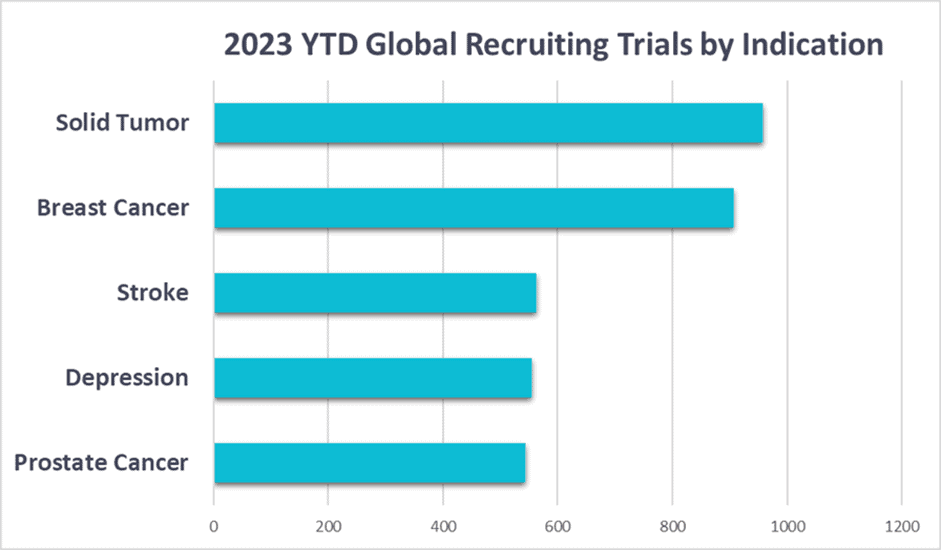
Fig.1. The top five most studied disease indications in the first six months of 2023
“Our new DEI Data Service was born of a real need for a more innovative solution to address the lack of diversity in clinical trials,” said Dr Gen Li, founder and CEO of Phesi. “Without diverse patient cohorts that accurately reflect the patient population a drug is intended for, we cannot ensure that drugs will be fully safe or effective. It’s clear the industry needs a new approach to deliver more equitable treatments, and our partnership with Krystelis will deliver this. The new service is the latest in our steps to disrupt clinical development and will enable clients to develop smarter trials and deliver faster cures.”
Krystelis’ expertise in medical writing services allows sponsors to embed diversity from the planning stage onwards, whether drafting protocols, applications for a new trial, regulatory documents or applications to market a drug. The DEI Data Service also leverages Phesi’s Digital Patient Profiles (DPP); each DPP provides a statistical view of patient attributes for a given disease, and provides a granular breakdown of the patient population, including attributes such as age, sex, ethnicity and comorbidities, among many other key variables. This insight enables sponsors to identify if a disease has a higher incidence in a particular patient population. For example, prostate cancer (one of the top five most studied disease areas in 2023 to date, see fig.1) has a significantly higher incidence and mortality rate in Black men than in White men – which should be reflected in patient cohorts.
“We are pleased to partner with Phesi to bring greater innovation to the issue of diversity in clinical trials,” Dr Pooja Phogat, co-founder and co-CEO of Krystelis. “The new service will make the development process easier for sponsors and help them to ensure they are meeting new, more stringent, regulatory requirements. It also has the potential to save sponsors millions of dollars and months of time, enabling them to bring more effective drugs to market, faster.”
Also commenting on the launch of the DEI Data Service was Otis Johnson, principal consultant, Trial Equity, “With the FDA issuing guidance to address failings in clinical trial diversity, sponsors are under increasing pressure to improve protocol design. It’s great to see companies like Phesi and Krystelis delivering practical solutions and services to help companies meet these new regulations quickly and effectively. Using data to improve inclusion in clinical trials brings us a step closer to a clinical development industry that benefits all patients.”
Phesi is attending the 13th annual DPHARM event held September 20-22. Phesi’s AI-driven platform Trial Accelerator, which simulates clinical trials and enables optimised protocol design, has been nominated for the DPHARM Idol Disrupt award. Phesi founder and CEO Dr Gen Li will be making a presentation at the conference and the Phesi team will also be available for meetings and demonstrations of Trial Accelerator at booth 62. Find out more/arrange a meeting: https://info.phesi.com/dpharm2023
About Phesi
Phesi provides comprehensive clinical development analytical products and services for biopharmaceutical companies around the world. Our integrated offerings cover the entire clinical development process – from development planning and indication assessment to protocol evaluation, site selection and trial implementation management. Visit: www.phesi.com.
About Krystelis
Krystelis was founded in 2022 by Dr Pooja Phogat and Dr Stuart Donald, two pharmaceutical industry executives with a combined 45 years of pharmaceutical industry experience, almost 30 years of this within service delivery organisations.
Their vision is for Krystelis to become a world-leading provider of high-quality, cost-effective services to the global life sciences industry. They are equally enthusiastic about exceeding the expectations of their customers and employees. As such, Pooja and Stuart are striving to make Krystelis not only great to work with, but also a great place to work.
The Krystelis team has decades of experience in successfully delivering services to global pharmaceutical companies. Working with Krystelis you can be confident that you will achieve high-quality outcomes that provide value for money. Our customers value our collaborative and proactive approach that helps them keep pace with rapidly changing regulatory requirements. Visit: www.krystelis.com.

